
Which is more viscous between glycerol and water based on their needs and intermolecular forces? As a result the glycerol molecules are highly associated and thus it has high viscosity. Glycerol undergoes extensive hydrogen bonding due to the presence of 3 –OH groups.
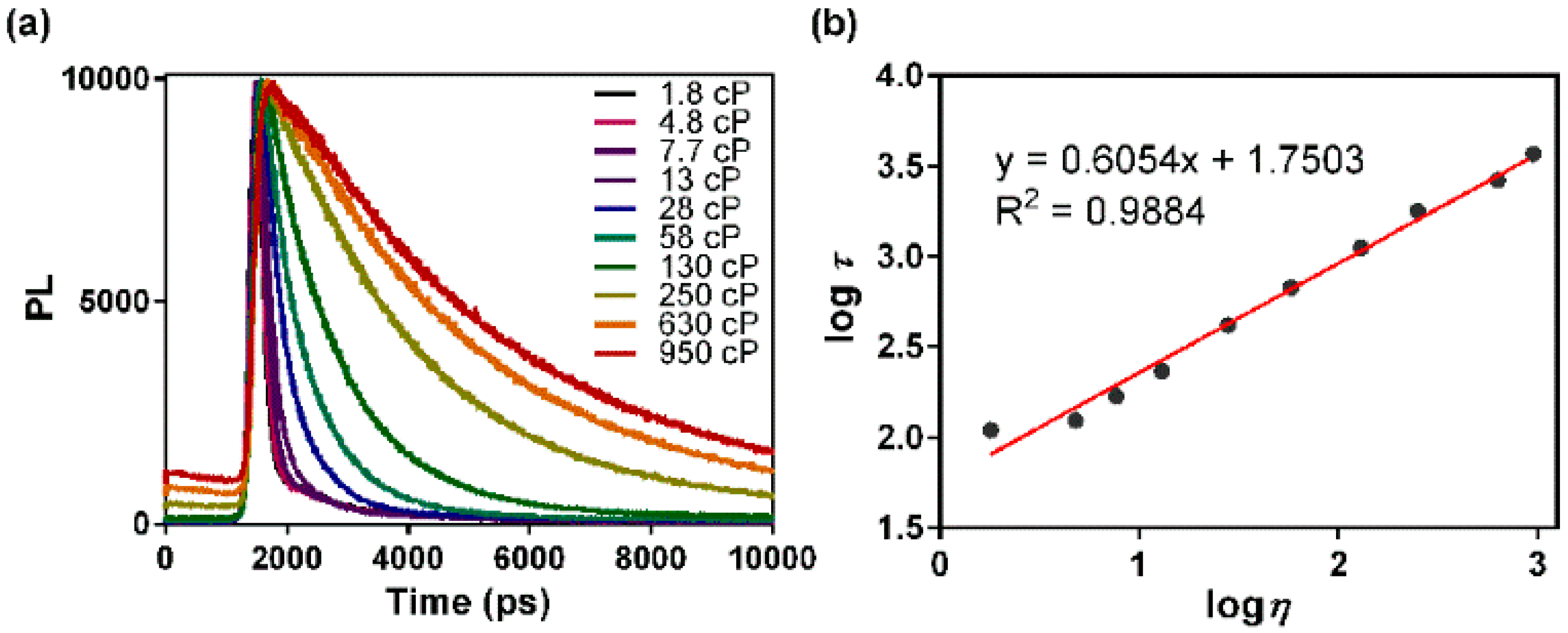
Was this answer helpful? Why Glycerine is viscous in nature? Alcohol also exhibit hydrogen bonding but glycerol contains three on per molecule and alcohol has only are OH group that can exhibit H−bonding. Glycerol is more viscous than ethane because of the expensive hydrogen bonding in glycerol. Why is glycerol more viscous than the other alcohols? That is why, glycerol has a higher viscosity than water. This means that the intermolecular forces are much greater because of these many hydrogen bonds. Which means to one molecule can atleast form 6 hydrogen bonds. Owing to the presence of three hydroxyl groups, glycerol is miscible with water and is hygroscopic in nature.Glycerol is has 3 OH groups. Increasing the glycerine concentration above 66.7 % will increase the freezing point as indicated below.
The freezing points are reduced until glycerine concentration is 66.7 % (mass). The boiling points of glycerine (also called glycerin or glycerol) water mixtures are reduced with increased amounts of glycerine.
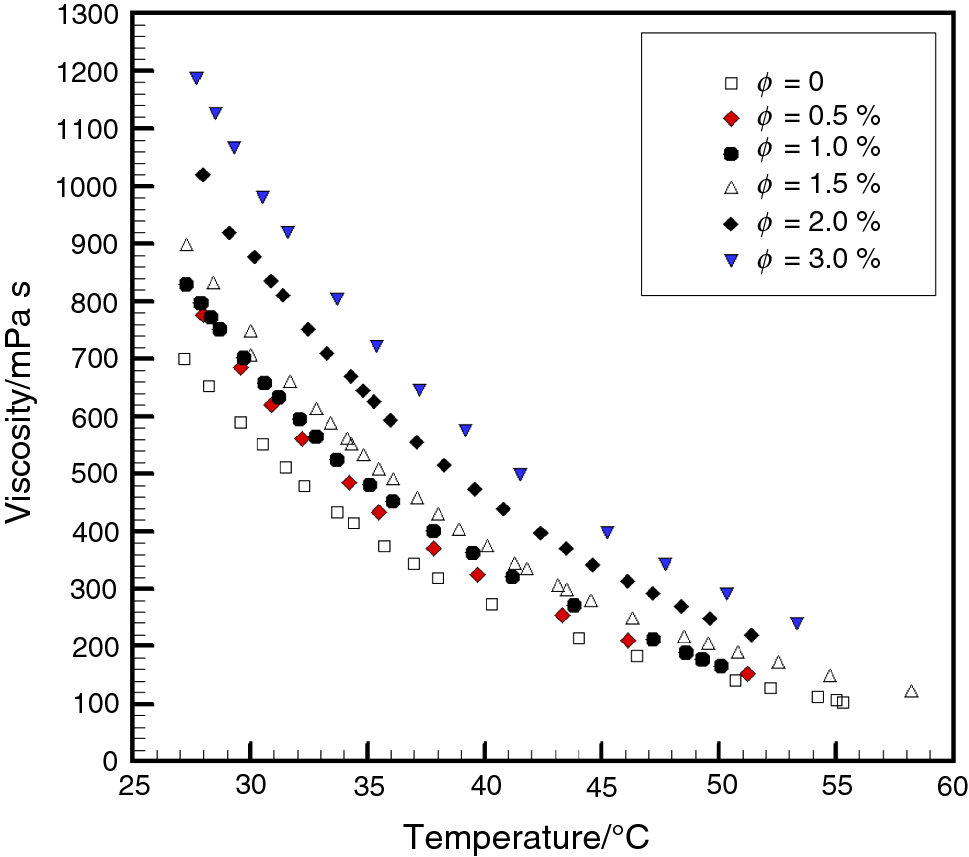
The temperature behaviour of glycerol has thought to be due to the existence of an extended hydrogen-bond network.

How does glycerol lower freezing point of water?Īs it is known, as cryoprotectant glycerol acts by stabilizing macromolecules, cells and tissues under cooling to subzero temperatures, along with suppressing the formation of ice. This abrupt difference in viscosity will directly influence the viscosity of water-glycerol solution, causing it to provide high values of viscosity. Water has a dynamic viscosity of 1.00 cP at 20 ☌, while the glycerol at the same temperature has a viscosity of 1,519 cP.


 0 kommentar(er)
0 kommentar(er)
